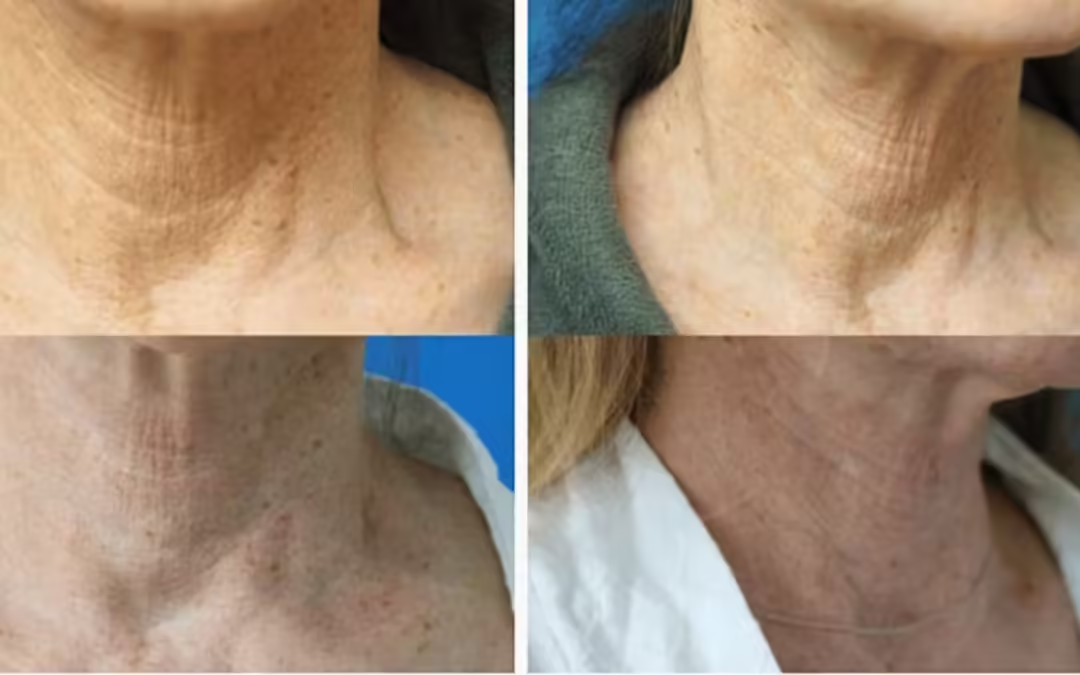
A new case study published in Cureus by Saami Khalifian, MD; Alec D. McCarthy, MD; and Steven Yoelin, MD, examines the efficacy and safety of an injectable therapy for treating neck wrinkles and skin laxity. This therapy combines hyperdiluted calcium hydroxylapatite (CaHA), platelet-rich plasma (PRP), and hyaluronidase.
Two patients with moderate neck wrinkles and skin laxity received the treatment and were evaluated several months later.
The study found that a single treatment with the combined therapy improved skin texture and laxity.
The inclusion of PRP and hyaluronidase aimed to enhance the regenerative effects of CaHA. PRP is rich in growth factors that stimulate collagen production and regeneration, while hyaluronidase breaks down hyaluronic acid, promoting better diffusion and even product dispersion.
The findings from these cases provide preliminary evidence supporting the safety and efficacy of this innovative combination therapy for addressing neck wrinkles and laxity.
According to the researchers, this is the first documented instance of using CaHA in conjunction with hyaluronidase and PRP for skin priming.
Further investigations are needed to explore the application of this treatment for other anatomical regions and to clarify the role of each injected component.
Read complete findings at Cureus
More from The Blog
Spring Holiday Promotions That Drive Revenue: How to Market Your Medspa Specials in the AI Era
Spring brings a cluster of gift-giving holidays that present golden opportunities for medspa owners – Easter, Mother's Day, and Father's Day all trigger increased purchasing behavior and active online searches for self-care and gifting options. In today's AI-driven...
Aviva Aesthetics Reveals Entrepreneur Equity: A Revolutionary Alternative to Traditional Private Equity for Med Spa Owners
As the aesthetics industry continues to mature, more med spa owners are considering their growth and exit strategies. With the AMSPA Conference 2025 kicking off tomorrow, we're breaking down one of the most innovative partnership models shaking up the industry – Aviva...
Salma Hayek Pinault Embraces Natural Beauty as New Face of Ultherapy PRIME
Academy Award-nominated actress Salma Hayek Pinault has made her first-ever venture into aesthetic partnerships, becoming the global brand ambassador for Ultherapy PRIME® – a strategic move that perfectly aligns with the industry's shift toward treatments that enhance...
Will Trump’s Tariffs Make Botox More Expensive? Here’s What Medspa Owners Need to Know
Trade wars aren’t just for economists and politicians anymore. With proposed import tariffs making headlines again in 2025, medspa owners have a new concern on their radar: Will neuromodulators like Botox, Dysport, and Jeuveau become more expensive? Short answer: Yes,...